The Ultimate Whiskey Ice Test
All photos and charts by Jake Emen.
An entire marketplace of products has rapidly risen to prominence, all promising to help you enjoy colder whiskey faster, or chilled whiskey without dilution. But do any of these products - from whiskey stones to huge ice spheres - actually work?
My first experience with whiskey stones had been... disappointing. Bringing in high expectations for the promised results, eventually I accepted reluctant defeat. In the years since, I passed on the bad news to any inquiring mind: "Sorry guys, I wanted to love the idea. Just didn't work."
But this is The Alcohol Professor. Let's bring some science into the equation shall we? If whiskey stones don't work, let's get the data to prove it. While we're at it, let's compare a dozen ice types, quantities and assorted chilling contraptions to see how they all stack up.
What follows is either the ravings of a madman, or a man just mad enough to channel his inner Walter White in the name of whiskey science.
The Whiskey Ice Experiment Setup
To pull this off correctly, the experiment had to be carefully controlled, minimizing as many variations as possible. Here are the basic rules I developed:
- After the 20 minute temperature check, the whiskey would be emptied into a beaker and its volume would be measured.
- After the volume measurement would be a quick taste test.
- Two whiskies* would be used, each going through the same 12 trials of different ice and chilling variations.
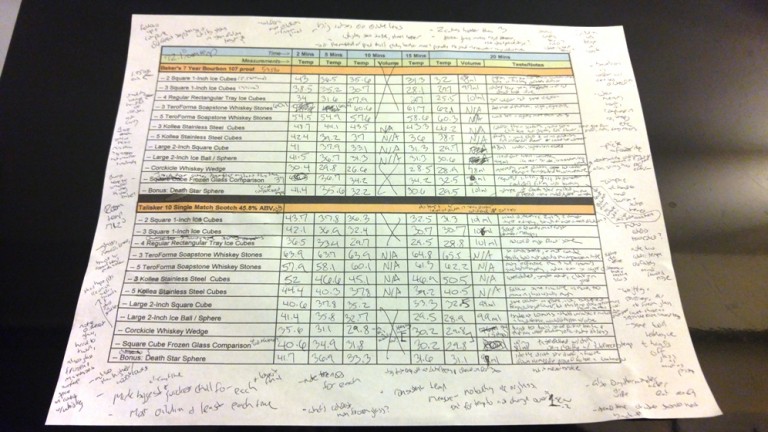
- With five temperature readings and one volume reading, that's a potential total of six measurements per trial, 72 per whiskey and 144 across both whiskies. In reality, the final number is actually 136 total data points, as volume readings are not applicable for stones which by design do not dilute.
*Why Two Whiskies? From an entirely unscientific perspective, it allowed your instructor to sample twice as much whiskey. But actually, the true purpose was determining whether or not the different whiskeys would experience different results. Put to the test were two excellent drams: Talisker 10 Year, a single malt Scotch at 91.6 proof, 45.8% ABV and Baker's, a 7 year old bourbon at 107 proof, 53.5% ABV. (Editor's note: Baker's won a silver medal in the 2015 NY International Spirits Competition.)
From these basic rules, things became progressively more rigid as I strived for maximum control and consistency. Before each temperature check, I would casually swirl the glass for exactly 10 seconds. This would simulate the experience of agitating the ice via sipping every few minutes.
During temperature checks, the thermometer was never touching the glass or any ice. The regimented swirls were performed with my fingertips just lightly grasping at the very top of the glass, and otherwise, I didn't touch or move the glass at all. The thermometer was dried and wiped clean between each measurement. The glass was washed out, and dried and returned to room temperature before being used again.
While originally I also wanted to measure volume and take a taste test at the 10 minute mark, I eventually canceled that strategy, realizing the taste test would distort volume, and pouring the whiskey in and out would distort both temperature, and timing. It had to be eliminated.
The starting room temperature of the whiskies also needed to be measured. Conducted on different nights, the Talisker Scotch tests began with a 71.2 degree room temperature, and the Baker's bourbon tests started at 72.1 degrees. It was worth noting the difference, but ultimately had no bearing on the results.
Dilution would be measured in milliliters for greater accuracy than trying to measure in ounces or tablespoons. While 2 ounces equals 59.1471 milliliters, any beaker aligns 60mls with 2 ounces. So for the final volume measurements, keep in mind a starting point of 60mls.
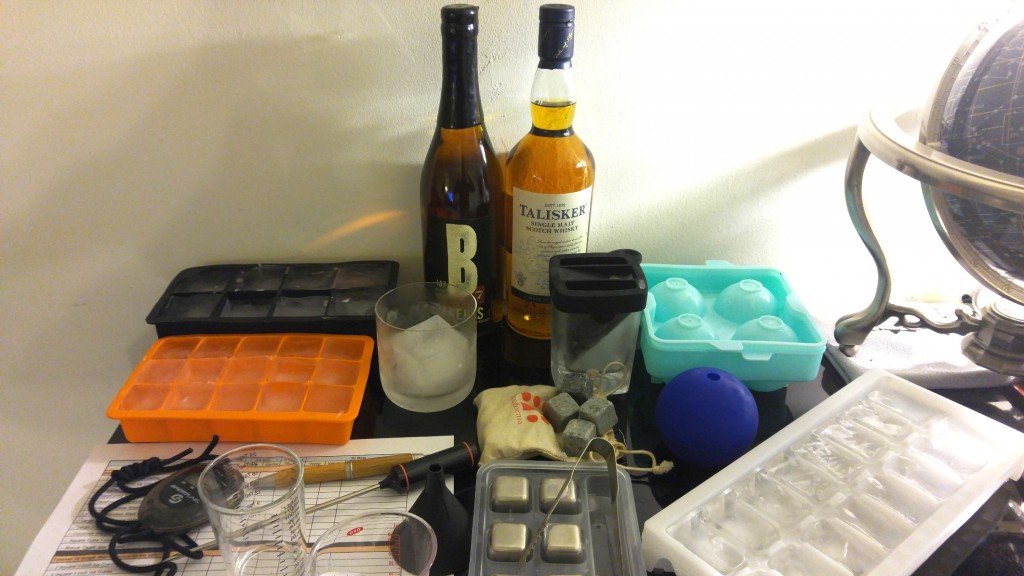
The Ice, Stones & Contraptions
- 4 regular rectangular ice tray cubes (1 ounce each = 4 ounces of ice)
- 3 TeroForma Soapstone Whisky Stones
- 5 TeroForma Soapstone Whisky Stones
- 3 Kollea Stainless Steel Cubes
- 5 Kollea Stainless Steel Cubes
- 1 large two-inch square ice cube (3.5 ounces)
- 1 large two-inch ice sphere (3.75 ounces)
- Corkcicle Whiskey Wedge
- Corkcicle Frozen Glass Comparison*
- Bonus Round: Death Star ice sphere**
*The Corkcicle Whiskey Wedge is in a category all of its own. Four ounces of water are poured through a mold which diagonally blocks off half the included glass which comes with the set. When frozen, the mold is removed, and you have the "wedge". However, in order to do this, the glass is frozen, creating an entirely different environment for chilling down a whiskey.
**Why? Because I have a Death Star ice sphere. And I use it frequently. Deal with it.
To attempt to gauge the difference this made, I ran two different comparisons. The first, tracked with the Talisker, involved using one of the large 3.5 ounce cubes, and pouring an extra .5 ounces of water into the glass to freeze along with it. Inexact, but it offered a matching total of 4 ounces of ice, along with a layer of ice solidly connected to glass, as opposed to floating around free. This didn't eliminate all variables, though. For instance, the Corkcicle glass itself is taller and narrower, with a lower volume capacity, than the more typical rocks glass used for all other tests.
For the second comparison, using the Baker's, 4 ounces of water were poured straight into the standard rocks glass being used. It created a solid layer of ice with the same volume as the wedge. Here, it was flat at the bottom of the standard glass, as opposed to diagonally affixed in the narrower Corkcicle glass.
The rest of the rig included two beakers, one for pouring a perfect starting 2 ounces, the other, a measuring beaker to view the final volume, with precise indicators up to 4 ounces. A culinary-grade thermometer was utilized, capable of going well above or below freezing with .1 degree accuracy, along with a stopwatch.
The Scientific Results & Analysis
How to interpret the chart: For each interval, and each whiskey, a coldest and warmest temperature is indicated, with blue text and red text, respectively. The overall coldest and warmest reading for each time, across both whiskies, is indicated via shading. As such, the overall lowest and highest marks at each interval are indicated with blue text on a blue background or red text on a red background. For the volume measurements, purple text indicates the least dilution, and gold text the most dilution, with shaded backgrounds once again indicating the overall leaders.
Major Takeaways & Trends
The Whiskey Matters – Here's Why
What became instantly clear was the divergence between the temperatures from the Talisker and the Baker's. Trends and patterns were consistent for each ice trial, but the bourbon was coming in colder. Different types of whiskey with different mash bills, sure, but the difference is in the proof, a substantial 15.4 margin between Talisker (91.6) and Baker's (107).
The conclusion drawn is that higher proof whiskey is both capable of being chilled quicker, and reaching lower overall temperatures. A higher proof means less water by volume, comparative to alcohol, in the glass.
Of 120 total temperatures, and 60 head-to-head comparisons, only in 12 instances was the higher proof bourbon warmer than the lower proof Scotch. That's 80% consistency, 48 to 12. However, let's take a closer look...
Five of those 12 warmer temperatures came from the different variation of Corkcicle glass comparison used, in which five for five, it led to higher temperatures (more below under Corckicle). Those two variations were too different to accurately compare head-to-head. So throwing out each set of temperatures, for a total of 110 readings and 55 head-to-heads, only seven were warmer for the higher proof bourbon, increasing the rate to 87%, 48 to 7.
Getting even more nitty-gritty, four of the remaining seven instances occurred in the Talisker ice sphere trial, in which the sphere broke (more below under ice sphere and mold variation) leading to increased chilling, while it didn't break in the Baker's trial. If you opted to toss out those as well, what remains is 50 head-to-heads across 10 full trials, and a 47 to 3 ratio, or 94% consistency that the higher proof bourbon was colder.
Does that Affect Dilution?
Overall, lower temperatures, reached at quicker rates, should deliver less dilution, as the ice is an environment less conducive to melting. However, with only eight head-to-head dilution comparisons, results were somewhat inconclusive, with the high proof, generally colder temperature bourbon achieving less dilution five times, more dilution twice, and an equal dilution once.
Explanations? In the two instances when the lower proof Scotch had less dilution, one was procued via variance, yet again, in the way one of the spheres melted, in this case the Death Star, and variance in the interaction of the whiskey with the Corkcicle led to the other.
Does Large Ice Does Dilute Less?
Results were inconclusive here. In the Talisker trial, all of the large ice varieties did indeed offer less dilution than their smaller counterparts, consisting of the two and three square 1-inch cubes, and the four rectangular cubes.
In the Baker's trial though, those results weren't reproduced. The large cube diluted equally to two square 1-inch cubes, and more than three square 1-inch cubes, and the Death Star sphere tied for the rectangular cubes with the largest level of dilution.
Whiskey Stones Still Don't Do Much
The original premise which led to this endeavor was reproduced and accurately recorded. The warmest temperature, at each interval, was with three TeroForma soapstone whiskey stones. The second warmest, at each interval, was with five of the soapstones. With temperatures consistently in the 60s, I noted the lack of chill to be somewhat startling in direct comparison to the others.
Steel Cubes Though...
The stainless steel cubes from Kollea performed significantly better than the soapstones, across each time, quantity and whiskey variety. In fact, the initial chilling effect of Kollea's steel cubes, measured at two minutes, was within range of many of the actual ice variations.
What remained true across either soapstone or steel cubes in any quantity was that over the course of 20 minutes, their temperature would consistently begin to tick back up, whereas with nearly every single ice trial, temperature continued to decrease. Even with the improved performance of the stainless steel cubes, they experience the same "rebounding" back to warmer temperatures.
Spheres & Molds Create Variance
Science doesn't like random chance, but spheres and their molds lead to it. In the first ice sphere trial, with Talisker, the sphere broke at the 10 minute mark, creating two smaller semispherical cubes in its place, and affecting temperature and dilution.
The Death Star didn't break in either trial – although I've witnessed that at other times – but it did melt in entirely different shapes in each instance, which also affected temperature and dilution. Like the original Death Star itself, for this ice mold in the Baker's trial, it seemed that a fissure in the middle of the mold left a weak point for the rebel forces to attack and expose, adding significantly more dilution.
Working with the spheres also produced to be a bit more difficult. With a hard plastic ice sphere tray, it was difficult to dislodge the spheres, while with the rubber plastic Death Star mold, it's a guessing game to make sure it's fully filled.
Your Whiskey Easily Gets Below Freezing
You can keep a bottle of booze in the freezer so that it's chilled, and it doesn't freeze on you, does it? So, it's not hard to put the pieces together here. Your whiskey can easily get below freezing, into at least the mid 20s. The lowest recorded temperature here was 25.5 degrees, measured at 20 minutes using Baker's bourbon and the four rectangular ice cubes.
The Corkcicle Effect & Comparisons
The Corkcicle delivered the quickest chill for both types of whiskey. In both instances, it was eventually overtaken for lowest temperature by the four regular rectangular ice cubes.
The Corkcicle also offered the least dilution for any form of ice, barring the second variation of the frozen glass comparison. Here, with 4 ounces of water frozen solid at the bottom, the spirit and the ice didn't interact as much --it couldn't be swirled to agitate the ice.
(Even less so than the Corkcicle itself, which also made swirling the whiskey very difficult. In the first Corkcicle trial, with the Talisker, the swirling was nearly impossible. I honed more of a swirling technique in the second trial, which led to the wedge melting much differently, and producing the variance which led to increased dilution mentioned above).
With the ice all frozen solid at the bottom of the glass, the top of the glass also warmed quicker to the touch than the entire Corkcicle glass did. Ultimately, this trial delivered the overall lowest dilution rate, showing that solid ice and a frozen glass lead to less dilution whether it comes in a Corkcicle box or not.
In the other Corkcicle comparison, in which the large two inch cube was frozen with .5 ounces of extra water attaching it to the frozen glass, some intriguing results were shown. It produced the same initial temperature reading as the two inch cube did on its own, but then began being substantially colder – 34.9 to 37.8, 31.8 to 35.2, 30.2 to 33.3, and 29.8 to 32.5.
Not surprisingly, a frozen glass, combined with ice which was connected to it rather than floating free, produced greater chilling. Most interestingly, the final two temperature readings for this frozen glass comparison and the Corkcicle were exactly the same – 30.2 degrees at 15 minutes and 29.8 degrees at 20 minutes. Both trials showed, in different ways, that you need not the Corkcicle to achieve its results -- it's the work of a frozen glass and solid ice.
Of Course There's More to Compare
Finally, did these experiments cover every type and quantity of ice, compared with every ratio of ice to booze, and every proof of alcohol? No. I went scientific on this, not crazy. But it did provide 136 data points delivered across a dozen trials each for two whiskies. Perhaps future experiments with different ratios of booze or new contraptions could lay ahead.
My Subjective View
The objective results and analysis above can be interpreted in myriad ways. Really, there are several factors to weigh in order to determine which choice will be best for you, because there's not a single, standalone correct answer. You have to determine your ideal level of chill and dilution, along with the experience you have while sipping, and weigh those factors against one another.
My bottom line is that coldest isn't best. While you probably didn't need a whiskey scientist to tell you this, the data allowed me to create a few demarcations. Once temperatures approach freezing, the flavor and aroma have both been very substantially dimmed. Even for someone looking to chill whiskey to make it friendlier, it's too cold to legitimately enjoy the flavors of the whiskey itself. At below freezing temperatures taste dulls even further, and once you get into the 20s, it's dead. Say goodbye; your flavor is gone.
Therefore, the two consistently coldest choices, the Corkcicle and the four regular rectangular ice cubes, were eliminated. For my preference, the Corkcicle, along with its frozen glass comparisons, would have been eliminated anyway. Holding a freezing cold glass just wasn't appealing in practice.
The most surprising revelation for me was the effectiveness of the Kollea stainless steel cubes, especially with five of them in the glass. They outperformed the soapstones by far, and offered a cold but not freezing temperature range. While five steel cubes is perhaps a bit clunky in the glass, the Kolleas will stay in my personal rotation when I want colder whiskey with no dilution. Otherwise, my choice is the large square 2-inch ice cube, easier to work with than ice spheres with comparable performance.
...But I'm still going to use the Death Star when I'm in the mood.